Send My Projects Request
Skaphor — CE FDA approved eye therapy device Category
Skaphor CE FDA approved eye therapy device Category
Overview — Trusted medical‑grade eye care
Skaphor, developed by Guangzhou Ruiheng Electronic Technology Co., Ltd. (founded 2018), offers a curated category of CE FDA approved eye therapy device solutions. As a national high‑tech enterprise focused on intelligent eye care, Skaphor combines rigorous R&D, manufacturing excellence and regulatory compliance to deliver reliable, safe devices for eye health management across more than 30 countries and regions.
Clinical-grade technology — evidence, safety and performance
Each CE FDA approved eye therapy device in the Skaphor portfolio is designed to meet medical standards and built on clinically informed engineering. Devices leverage medical‑grade components, non‑invasive modalities and AI‑driven features to support precise treatment protocols and user monitoring. Our products undergo strict quality control and regulatory certification processes to ensure safety and repeatability for professional and at‑home settings.
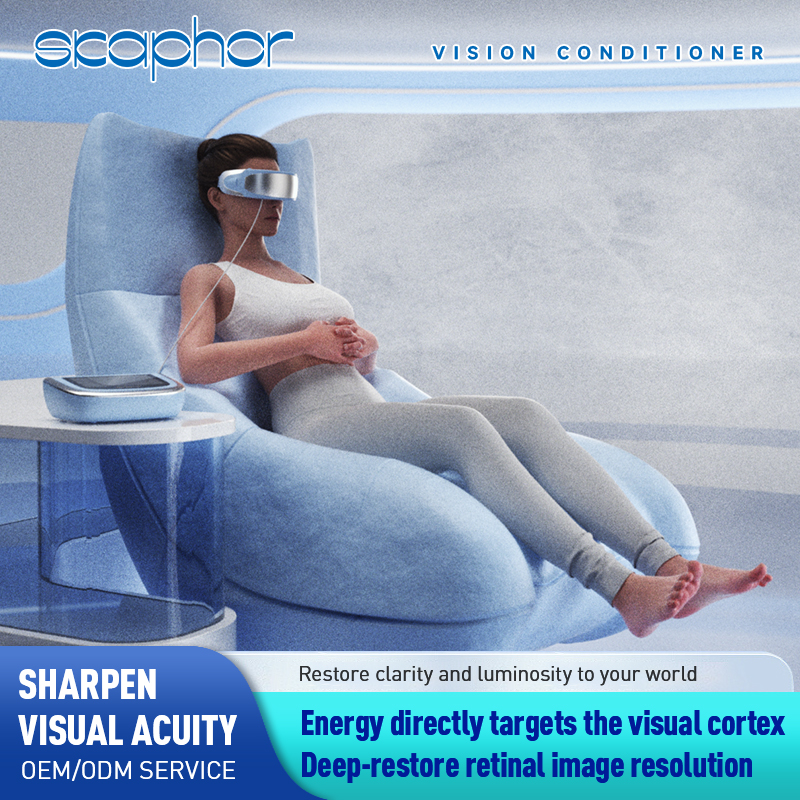
Applications & benefits — practical eye health solutions
Skaphor devices are intended to support eye comfort, recovery and ongoing management by offering targeted therapies for symptoms such as eye fatigue and surface discomfort. Designed for ophthalmic clinics, optometry practices and everyday wellness users, our CE FDA approved eye therapy device range emphasizes ease of use, data‑driven personalization and measurable outcomes that help professionals and consumers implement consistent eye care routines.
Why choose Skaphor — experience, innovation and global support
As an innovative leader in global eye care, Skaphor blends more than half a decade of specialized R&D with end‑to‑end manufacturing and international distribution. Our mission — scientific eye care, guarding eyesight — drives continuous improvement, backed by robust documentation, technician training and customer support. Choose Skaphor for proven compliance, AI‑enhanced functionality and a partner committed to advancing safe, effective eye care technologies worldwide. Visit https://www.skaphor.net/ to learn more.
CE FDA approved eye therapy device Display
Do you provide after-sales spare parts inventory?
For VIP customers to keep three per thousand of the order quantity of spare machine inventory (free hosting for 1 year).
Is there any exclusive logistics program for large orders?
More than 500 units, we can provide dedicated logistics (including export customs clearance/destination country customs clearance).
What support do you offer after purchase?
We provide full technical support, user manuals, and responsive after-sales service. Warranty policies and remote assistance are available to ensure your satisfaction.
Do you offer OEM or ODM services?
Absolutely. We have a complete and mature OEM/ODM system and welcome global partners to customize products with your branding and requirements.

Skaphor Vision Revival Device - Eye Therapy Device for Visual Acuity & Dry Eyes (0-100Hz Bio-Optical)
Skaphor Vision Revival Device - Eye Therapy Device for Visual Acuity & Dry Eyes (0-100Hz Bio-Optical)© 2026 Skaphor. All Rights Reserved.



zhu Juliy
Skaphor_ Juliy